Custom Analysis Organization
Home » CAO
A complete testing strategy
Ironclad security of results, quick execution time and cost-effectiveness, these are the primary reasons that drive our customers to rely on us.
The importance of an optimal testing strategy
Our laboratories carry out Custom Made testing strategies specifically created around the peculiarities and needs of each individual tested product.
Testing on volunteers only as confirmation of in vitro tests
Volunteer testing is only used as a confirmation of the safety and effectiveness resultsobtained by the in vitro tests in the testing strategy.
This allows us to reduce the risk for volunteers compared to other practices established in the industry.
In verifying the safety of your product C.A.O. laboratories implement the best and most advanced in vivo and in vitro testing strategies to ensure the best analysis service on the market.
In order to evaluate the effectiveness of products and devices intended for humans and study their mechanisms of action, C.A.O. makes the most of its network of international Partner Labs.
Why decide for yourself if your customers will be the ones buying?
Our professional consultants will work with you to reach your goals. They will help you define your communication and brand strategy for optimal marketing performance.
The consumer panel of our Beauty & Wellness Communityis ready to test your product before it gets to market.
Our strategies
The eye is an extremely delicate organ.
Cosmetic products that may come into contact with it must be thoroughly evaluated, from a safety standpoint, by the Safety Assessor.
The testing of possible dermal irritation caused by a cosmetic product, among the evidence that the Company must produce for its Product Information File, is of fundamental importance.
Ethical models, new tests
INT.E.G.RA. is dedicated to the research, development and validation of in vitro tests to determine the beneficial effects of cosmetics: rejuvenating, healing and antioxidants.

Our tests
Alternative tests
In vitro skin irritation test, on the samples provided, using the Dermal IRRITECTION® Method
In vitro eye irritation test, on samples provided, using Protocol DB-ALM 157
IRRITECTION®, OECD APPROVED 496.
In vitro eye irritation test. Ocular IRRITECTION® Protocol.
Dermal Corrosivity in vitro test. Corrositex®; OECD 435 Protocol.
Vitality tests on various celliular lines (HaCaT, HECV, macrofagi-raw 264.7, CCD-1070Sk, MCF7, FaO).
Evaluation of the skin irritant effect on the skin using reconstituted skin according to OECD Guideline Protocol 439.
Laboratory tests
Nickel,GF-AAS Lead,AAS Cadmium,AAS Zinc,FL-AAS,Cobalt,FL-AAS,FL-AAS Titre
Cromo,GF-AAS, titolo Mercurio, AAS-CVG, titolo.
Evaluation of the maintenance over time of the prerogatives of the product for the use for which it was formulated.
Stress conditions: in thermostat (45°C); in refrigerator ( 2-4°C); at room temperature (25°C).
Main phenomena evaluated: Creaming; Flocculation; Coalescence; Phase inversion; Packaging deformation.
Other analylses available on request: pH measurement; Viscosity measurement; Light stability; Thermal shock.
The analysis has a duration of at least one month with deadlines distributed over time.
The method is based on the determination of the D value, defined as the time, expressed in hours, necessary to reduce the inoculated mycorbic charge by 1 logarithm (90%).
The inoculum is made of multiple microbial strains, gram positive, gram negative, yeast and mold, as in a classic challenge test. the D value is then corrected according to an index given by the sum of two factors: the type of packaging in which the product is packaged and the type of use expected for the product.
consumer.
This variation of the method also makes it possible to judge the shelf life of the product after it has been opened and during use, to suggest suitable packaging or instructions for use and appropriate post-opening expiry dates based on the specific D values found.
Samples Required: 1 sample of 150 ml + INCI Run time: 3 days per inoculum 28 days incubation 3 days for reading.
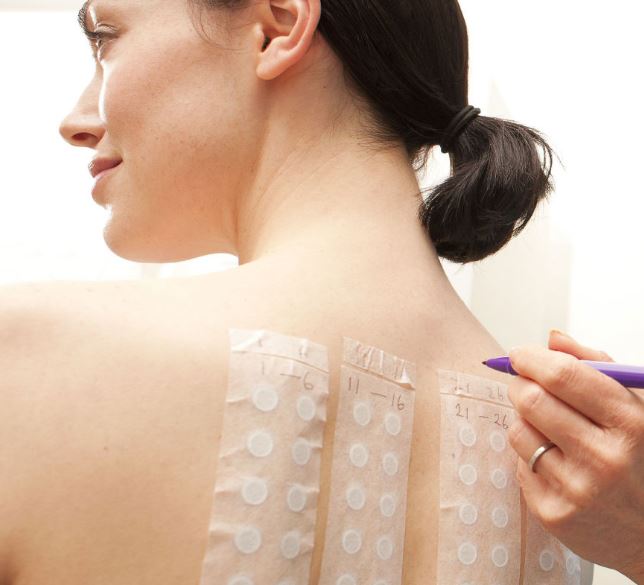
Patch test on volunteers.
Microbiome tset with our partner Kind To Biome.
Claim substantiation test.
Consumer tests
Likeabiliity and expected purchase propensity (price being known) of my product, based only on the “concept” and sales price established.
– Gradimento atteso del mio prodotto vs il competitor.
– Propensione all’acquisto attesa (noto il prezzo) del mio prodotto vs competitor.
– Comparison of my product with the competitor, based only on the “concept” and established selling price of my product.
– Expected and real enjoyment of my product.
– Expected and real purchase propensity (the price is known) of my product.
– Perceived product brand identity.
– Product’s warning points.
– Packaging profile of my product, based on 160 samples of the product in real sales packaging.
– Gradimento atteso e reale del mio prodotto e del competitor.
– Expected and real purchase propensity (price is known) of my product and the competitor’s.
– Distance of my product from the competition.
– My product’s perceived brand identity vs. the competitor’s.
– Warning point of my product and competitor’s.
– My product’s packaging likeability vs the competition’s.
Basato su: 160 campioni del tuo prodotto + 160 campioni del prodotto del tuo concorrente in confezioni reali di vendita.
Complete list of tests
| QC batch release test for determination of ethanol title |
| In vitro Protocol Ocular IRRITECTION |
| In vitro Ocular Irritation OECD TG 496 |
| Ocular Irritation Package: preliminary in vitro + ophthalmological test on volunteers in vivo |
| In vitro skin irritation Dermal IRRITECTION Protocol |
| Skin Irritation Package: preliminary in vitro + dermatological patch test on in vivo volunteers |
| In vitro skin irritation OECD TG 439 protocol |
| In vitro skin corrosivity OECD TG 435 |
| Challenge test according to EU Pharmacopoeia |
| Microbiological analysis of cosmetic products |
| Verification of stability/compatibility of the cosmetic product |
| Microbiological stability analysis and predictive information for PAO (Period After Opening) |
| Heavy metals, 5 determinations (Pb, Cd, As, Hg, Cr) |
| Allergen determination |
| Preservatives: HPLC analysis |
| UV filters: HPLC analysis |
| Dyes: HPLC analysis |
| Dioxane: residual solvent analysis |
| Benzene: Residual solvent analysis |
| Consumer Test type 1: Entry level my product/my concept price test |
| Consumer Test type 2: my competitor product |
| Consumer Test type 3: my product |
| Consumer Test type 4: Actual rating (my product vs. the competitor) |
| SPF efficacy in vitro, according to the DIFFEY method |
| Other skin efficacy tests on volunteers with dermatologist supervision |
| Other trichological efficacy tests on volunteers under the supervision of a dermatologist |
| Other oral efficacy tests on volunteers supervised by a dentist |
| Other ocular efficacy tests on volunteers with ophthalmologist supervision |

